Pipeline targeting rare kidney and metabolic disease
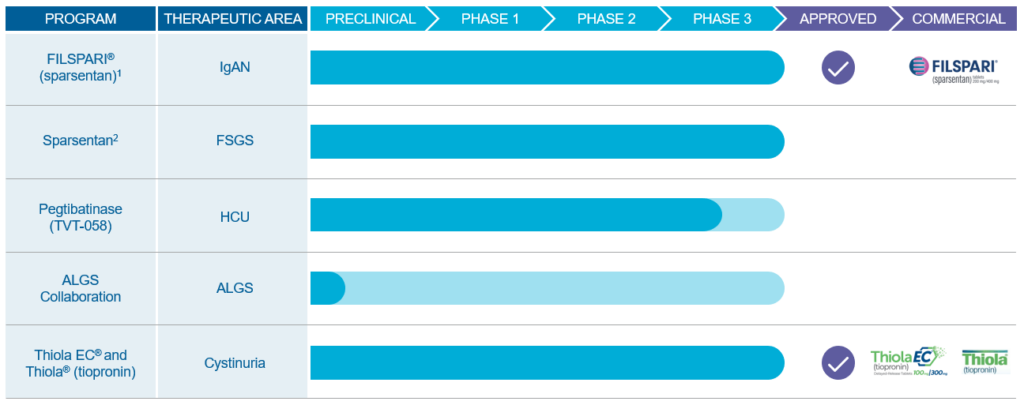
1 In February 2023, the Company announced that the FDA granted approval of sparsentan under the accelerated approval pathway for the reduction of proteinuria in IgAN. In September 2023, the Company announced confirmatory data from the Phase 3 PROTECT Study of FILSPARI®. Read the press release> CSL Vifor has exclusive commercial rights for sparsentan in Europe, Australia, and New Zealand. Read the press release> Renalys Pharma has exclusive commercial rights for sparsentan in Japan, South Korea, Taiwan, and Southeast Asian nations. Read the press release>
2 In December 2023, the Company announced that following its engagement with FDA on the two-year results from the DUPLEX Study, the Company is conducting additional analyses of FSGS data, and plans to re-engage with the FDA later in 2024 following the Company’s consideration of additional evidence. Read the press release>
FSGS: focal segmental glomerulosclerosis; IgAN: IgA nephropathy; HCU: classical homocystinuria; ALGS: Alagille syndrome